The pharmaceutical industry is constantly evolving, and one of the latest developments is the rise of biosimilars. While biosimilars are often compared to generic drugs, they are fundamentally different. In this article, we will explore the differences between biosimilars and generics, and how biosimilars are changing the landscape of the pharmaceutical industry and improving patient outcomes.
The Difference Between Biosimilars and Generics
A. Definition of Generic Drugs
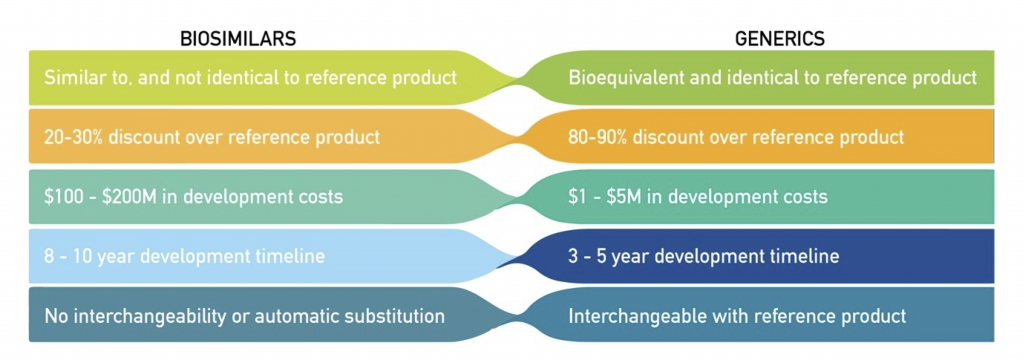
Generic drugs are identical copies of the original drug, also known as the reference drug. They contain the same active ingredients, dosage, and strength as the original drug. Generic drugs are produced after the patent of the original drug expires, allowing other manufacturers to produce and sell the same drug at a lower cost, making it more affordable and accessible for patients who cannot afford the original drug.
B. Definition of Biosimilars
In contrast, biosimilars are not identical copies of the reference drug. They are highly similar but not identical, as they are produced from living cells and are more complex in their molecular structure. Biosimilars are designed to be highly similar to the original drug, but not identical, as it is not possible to produce an exact copy of a biological molecule. And below is a summary of the differences between biosimilars and generics.
C. How the Approval Process Differs
The approval process for biosimilars is much more rigorous compared to generic drugs. Biosimilars undergo clinical trials to demonstrate their safety and efficacy, unlike generic drugs. They are also subject to additional regulations, such as post-market monitoring, to ensure patient safety.
How Biosimilars Are Changing the Landscape of the Pharmaceutical Industry
A. Increased Affordability
Biosimilars offer a more affordable alternative to expensive biologic drugs. Biologic drugs are often used to treat chronic conditions such as cancer, rheumatoid arthritis, and diabetes, and can cost thousands of dollars per treatment. Biosimilars can provide the same benefits at a lower cost, making them more accessible to patients who cannot afford the original biologic drug. This increased affordability allows more patients to access the medications they need to treat their conditions.
B. Increased Competition
Biosimilars can increase competition in the market, driving down the prices of biologic drugs. With more companies producing biosimilars, there is more competition in the market, leading to lower prices for biologic drugs. This can benefit both patients and healthcare systems, as it reduces the burden of healthcare costs.
C. Improved Patient Outcomes
Biosimilars can improve patient outcomes. With more affordable alternatives to biologic drugs, patients can access treatments that they may not have been able to afford before. This can lead to better health outcomes and improved quality of life for patients. In addition, biosimilars also foster innovation and deliver other non-price-driven advantages. By reducing financial barriers to biological therapies, biosimilars play a part in budgetary redistribution and, hence, in increasing patients’ access to treatment.
Examples of Biosimilars Making an Impact
Biosimilars for Rheumatoid Arthritis Drugs
Biosimilars for drugs such as Humira and Remicade, used to treat rheumatoid arthritis, have been approved in Europe and the US, providing more affordable options for patients. These biosimilars have demonstrated similar efficacy and safety profiles to the original drugs, making them a viable treatment option for patients.
Biosimilar of Herceptin for Breast Cancer Treatment
In India, a biosimilar of Herceptin, a breast cancer drug, was approved in 2014 and has since been used to treat over 30,000 patients. The biosimilar is significantly more
Conclusion:
In conclusion, biosimilars are similar but not identical to the original biologic drug and offer a more affordable alternative to expensive biologic drugs. Biosimilars have the potential to increase competition, lower healthcare costs, and improve patient outcomes. With the rise of biosimilars, we can expect to see a shift in the pharmaceutical industry towards more accessible and affordable treatments for patients. As biosimilars continue to gain approval and market share, the healthcare industry can expect to see significant changes in both drug pricing and patient outcomes.

